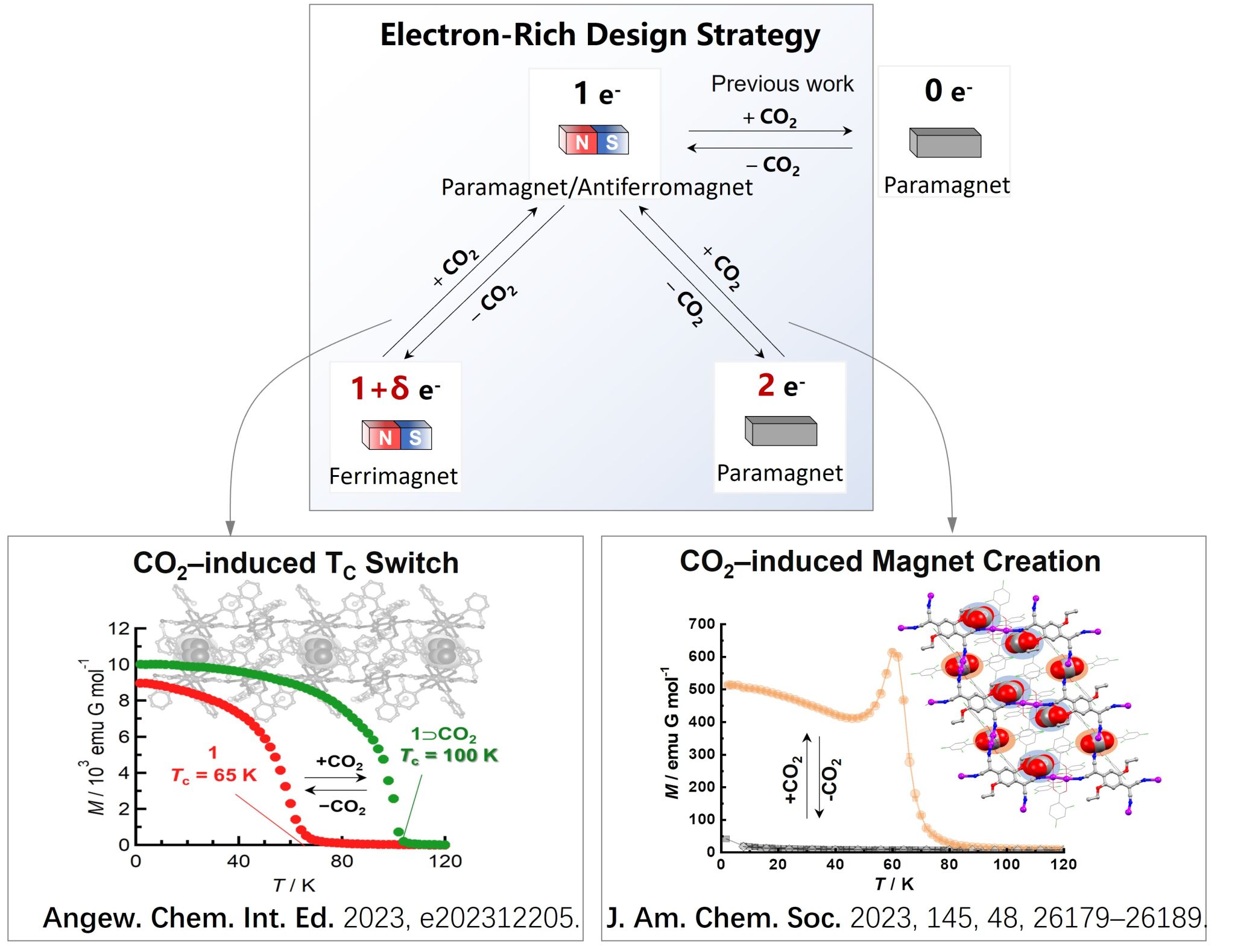
Recently, Professor Jun Zhang from the School of Chemistry and Molecular Sciences at Wuhan University, in collaboration with Professor Hitoshi Miyasaka from Tohoku University and Professor Yasutaka Kitagawa from Osaka University, proposed an innovative "electron-rich design strategy." This approach has successfully utilized carbon dioxide (CO2) to regulate the electron and spin states of a series of multi-electron transfer-type porous molecular magnets. These findings, showcasing reversible transitions of Curie temperature and magnetic states have been published in Angewandte Chemie International Edition and the Journal of the American Chemical Society (JACS).
Magnetic materials play a crucial role in modern industry and technology. Unlike traditional magnets, molecule-based magnetic materials can be precisely designed and constructed at the molecular level, promising advancements in high-density data storage and quantum computing. Porous magnets, combining chemical porosity with physical magnetism, can regulate their electronic structure through the adsorption of guest molecules (solvents or gases), enabling precise magnetic control. Due to the rapid adsorption and desorption and minimal structural damage, gases are particularly effective for this purpose. However, the interaction between gases and porous magnets is typically weak, resulting in negligible magnetic changes. Hence, designing and precisely controlling the structure of porous molecular magnets to achieve diverse magnetic phase transitions remains a significant scientific challenge.
Professor Zhang has long been dedicated to the construction and regulation of the physical properties of redox-type porous magnets, focusing on functional conversions of magnetic, electrical, and optical properties through guest molecules. The team has constructed various electron transfer-type porous magnets, proposing mechanisms like host-based electron transfer, host-guest electron transfer, and host-guest spin coupling, achieving reversible magnetic phase transitions. Their earlier work reported CO2-induced changes in the long-range magnetic order of porous magnets, achieving demagnetization switching from ferrimagnetic to paramagnetic states.
To address the aforementioned challenges, Professor Zhang's team, in collaboration with Japanese researchers, introduced the "electron-rich design strategy" for synthesizing multi-electron transfer-type porous magnets. They first synthesized a 1+δe- (0<δ<1) transfer-type porous magnet with ferrimagnetic properties (Curie temperature Tc = 65 K). Upon CO2 adsorption, the electron transfer number changed to 1e-, significantly altering Tc (Tc = 100 K). This study demonstrated that CO2 adsorption effectively inhibits electron transfer, achieving reversible Tc conversion while maintaining structural stability. This breakthrough was published in Angewandte Chemie International Edition and selected as a "Hot Paper."
Additionally, the "electron-rich design strategy" was applied to design and synthesize a 2e- transfer-type porous magnet exhibiting paramagnetic behavior. After CO2 adsorption, the Ru(II) dimer's electron state transitioned from Ru2IIIII to Ru2IIII, changing the electron transfer number from 2e- to 1e-, successfully observing the transition between paramagnetic and antiferromagnetic states. This pioneering research on CO2-induced magnet creation was published in JACS.
Article information:
1. Wataru Kosaka, Yoshie Hiwatashi, Naoka Amamizu, Yasutaka Kitagawa, Jun Zhang, Hitoshi Miyasaka*, Densely Packed CO2 Aids Charge, Spin, and Lattice Ordering Partially Fluctuated in a Porous Metal-Organic Framework Magnet. Angew. Chem. Int. Ed. 2023, e202312205.
2. Jun Zhang, Wataru Kosaka, Qingxin Liu, Naoka Amamizu, Yasutaka Kitagawa, and Hitoshi Miyasaka*, CO2-Sensitive Porous Magnet: Antiferromagnet Creation from a Paramagnetic Charge-Transfer Layered Metal-Organic Framework. J. Am. Chem. Soc. 2023, 145, 48, 26179–26189.
Copyright 2024 © Zhang Lab at College of Chemistry and Molecular Sciences of Wuhan University